Now in its 5th year, the iPSC Drug Development Summit is the longest-standing and most comprehensive meeting focused exclusively on iPSC-derived therapies. The Summit returns to Boston at a pivotal time for the field, reflecting the community’s rebound with renewed clinical momentum and innovation. This year introduces two brand-new tracks: Preclinical Development, covering early-stage challenges like genetic engineering and culture optimization, and Process Development & CMC, addressing scale-up, reproducibility, and product quality.
Along with 2 new can’t-miss workshops on refining starting cell line selection and aligning on industry-wide QC and differentiation standards, crucial for reducing risk and accelerating clinical success. With progress from leaders like Aspen Neuroscience, Century Therapeutics, and BlueRock Therapeutics, this is your go-to forum to connect with the full iPSC community, learn from those advancing into the clinic, and ensure you’re part of the next iPSC boom.
What DIFFERENTIATES the 5th iPSC Drug Development Summit?
Enhancing understanding of starting cell line selection by exploring donor variability and predictive assays with iPSirius and Thymmune Therapeutics
Defining necessary steps to advance into clinical development through enforcing GMP grade processes meeting regulatory requirements with lessons learned from Novo Nordisk and a case study from Century Therapeutics
Maximizing the scalable expansion of high quality and consistent iPSC-derived cells by exploring 2D versus 3D platforms and integrating automation with BlueRock and HebeCell
Advancing immune reconstitution, cancer therapy and more by examining engineered iPSC-derived cells and allo-evasion techniques with Lift Biosciences and Kenai Therapeutics
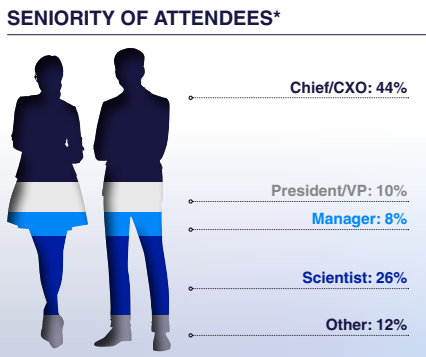
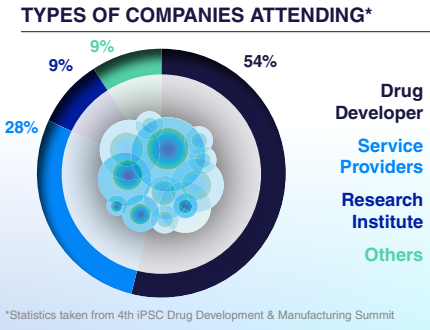
Who Will You Meet?
This is your opportunity to connect with likeminded peers and top pioneers in the iPSC based therapies space from R&D and preclinical directors to process development, CMC, manufacturing, and regulatory experts.